Recently, AstraZeneca reached an acquisition agreement with CinCor for an estimated total purchase price of US$1.8 billion (RMB12.1 billion), which is the largest acquisition by AstraZeneca since the beginning of the year.
Why is AstraZeneca "willing" to spend $12.1 billion in the face of the capital chill?
Who is CinCor?
CinCor is a US-based clinical-stage biopharmaceutical company focused on the development of novel treatments for resistant and uncontrolled hypertension and chronic kidney disease.
Under the terms of the agreement, AstraZeneca will launch an offer to acquire all outstanding shares of CinCor at a purchase price of US$26 per share at closing, with AstraZeneca paying a further US$10 per share in non-tradable contingent value rights (CVR) upon submission of the baxdrostat product to certain regulatory authorities. The upfront cash component of the transaction is approximately $1.3 billion, representing a 121% premium to CinCor's closing price on January 6, 2023.

Together, the upfront payment and the largest potential contingent value payment will bring the value of the transaction to US$1.8 billion, a 206% premium to CinCor's closing price on 6 January.
This time, AstraZeneca will strengthen AstraZeneca's cardio-renal pipeline by acquiring CinCor's drug candidate baxdrostat (CIN-107).
baxdrostat is an aldosterone synthase inhibitor (ASI) used to lower blood pressure in treatment-resistant hypertension.
Baxdrostat represents a potential leading next generation ASI, a highly selective oral small molecule inhibitor of aldosterone synthase, the enzyme responsible for aldosterone synthesis in the adrenal glands, being developed for patient populations with significant unmet medical needs, including therapeutic hypertension, primary hyperaldosteronism and chronic kidney disease.
Baxdrostat selectively targets the aldosterone synthase enzyme encoded by the CYP11B2 gene and has a much lower affinity for the blocking activity of the 11ß-hydroxylase encoded by the CYP11B1 gene responsible for cortisol synthesis.
In its phase 2 clinical trial, Baxdrostat significantly reduced systolic blood pressure after 12 weeks and significantly reduced aldosterone levels across a wide range of doses without affecting cortisol levels.
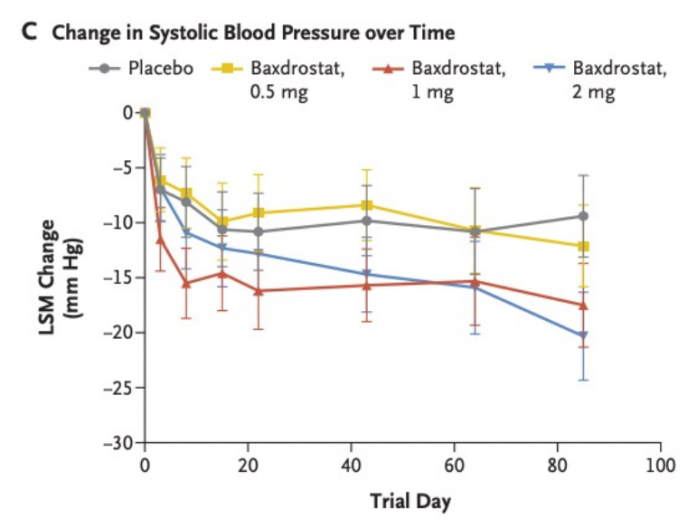
Figure 1 Baxdrostat Phase 2 clinical trial results
AstraZeneca is on the move
In addition to this acquisition, AstraZeneca has spent approximately US$40 billion on cardiovascular disease acquisitions in six transactions over the last four years.
During this period, AstraZeneca has also been involved in five separate oncology and respiratory disease deals. These are important steps in the company's plans to expand its portfolio.
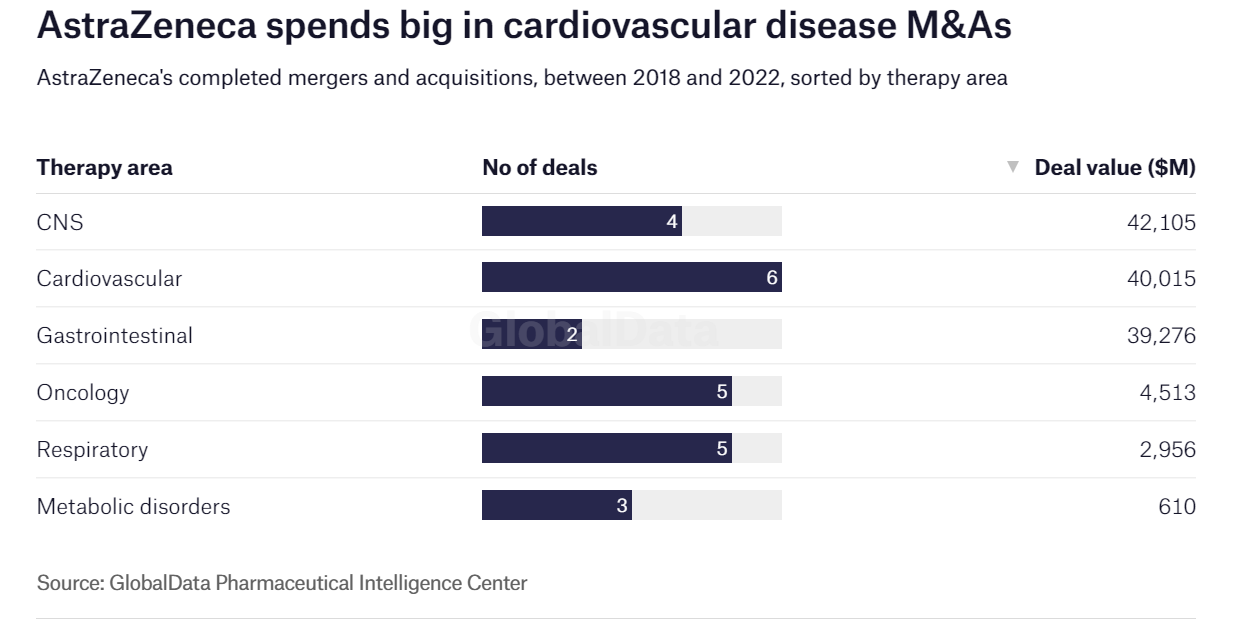
Figure 2 Acquisitions completed by AstraZeneca between 2018 and 2022
An example of AstraZeneca's deals in the clinical area of cardiovascular disease is its agreement with Dogma Therapeutics in the US.
In 2020, AstraZeneca acquired Dogma Therapeutics' preclinical oral PCSK9 inhibitor programme. PCSK9 inhibitors regulate low-density lipoprotein (LDL) levels to lower LDL cholesterol. Elevated LDL cholesterol levels are a major risk factor for cardiovascular disease.
In addition to the cardiovascular system disease aspect, the acquisition of Alexion Pharmaceuticals in 2021, a transaction in which AstraZeneca was required to pay US$13.3 billion in cash and certain equity interests to the other shareholder. In the same year, AstraZeneca entered into a global development and commercialisation agreement with Ionis Pharmaceuticals to investigate Eplontersen for the treatment of familial amyloid neuropathy (FAN) and familial amyloid cardiomyopathy (FAC).
In July 2022, AstraZeneca acquired TeneoTwo, including its clinical-stage CD19/CD3 T-cell inducer TNB-486.The AstraZeneca acquisition accelerates the development of new B-cell haematology malignancies in which TNB-486 is available, including diffuse large B-cell lymphoma and follicular lymphoma.TNB-486 further enriches AstraZeneca's haematology pipeline, which spans multiple therapeutic modalities and mechanisms to address a broad range of blood cancers. The value of the deal could reach US$1.27 billion.
How is the self-developed pipeline progressing?
In recent years, the focus areas of AstraZeneca's pipeline have included oncology, biopharmaceuticals (including cardiovascular, renal and metabolic, respiratory and immunology) and rare disease drug development, with anti-tumour being the most important area of investment.
According to the company's website, there are currently 179 projects in development, including 13 new molecular entities in advanced stages of development.
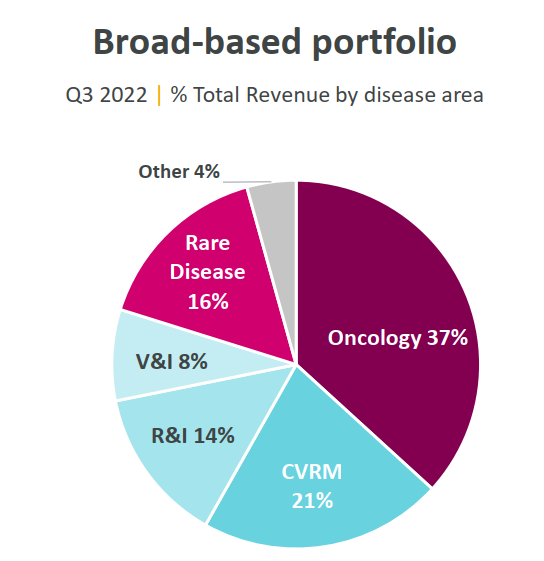
Figure 3 Disease areas of focus for AstraZeneca
Anti-tumour area
AstraZeneca aims to expand treatment options and improve outcomes for patients with solid tumours and haematological cancers. The company focuses on tumour drivers and resistance, immuno-oncology, DNA damage response, antibody drug conjugates and epigenetic and cellular therapies, while advancing innovative clinical strategies to treat patients in the early stages of disease and those who are relapsed or refractory to treatment.
Currently, there are 10 projects in clinical phase 1, 11 in phase 2 and 17 in phase 3. Several products have progressed positively in the last year, including.
1) Capivasertib CAPItello-291, a potent oral Akt1/2/3 inhibitor developed in-house by AstraZeneca. In a Phase 3 clinical trial, Capivasertib+fulvestrant showed a statistically significant improvement in PFS in the overall and AKT pathway altered population significance and clinical significance, achieving a dual primary focus, and safety with a relatively low rate of discontinuation due to adverse events.
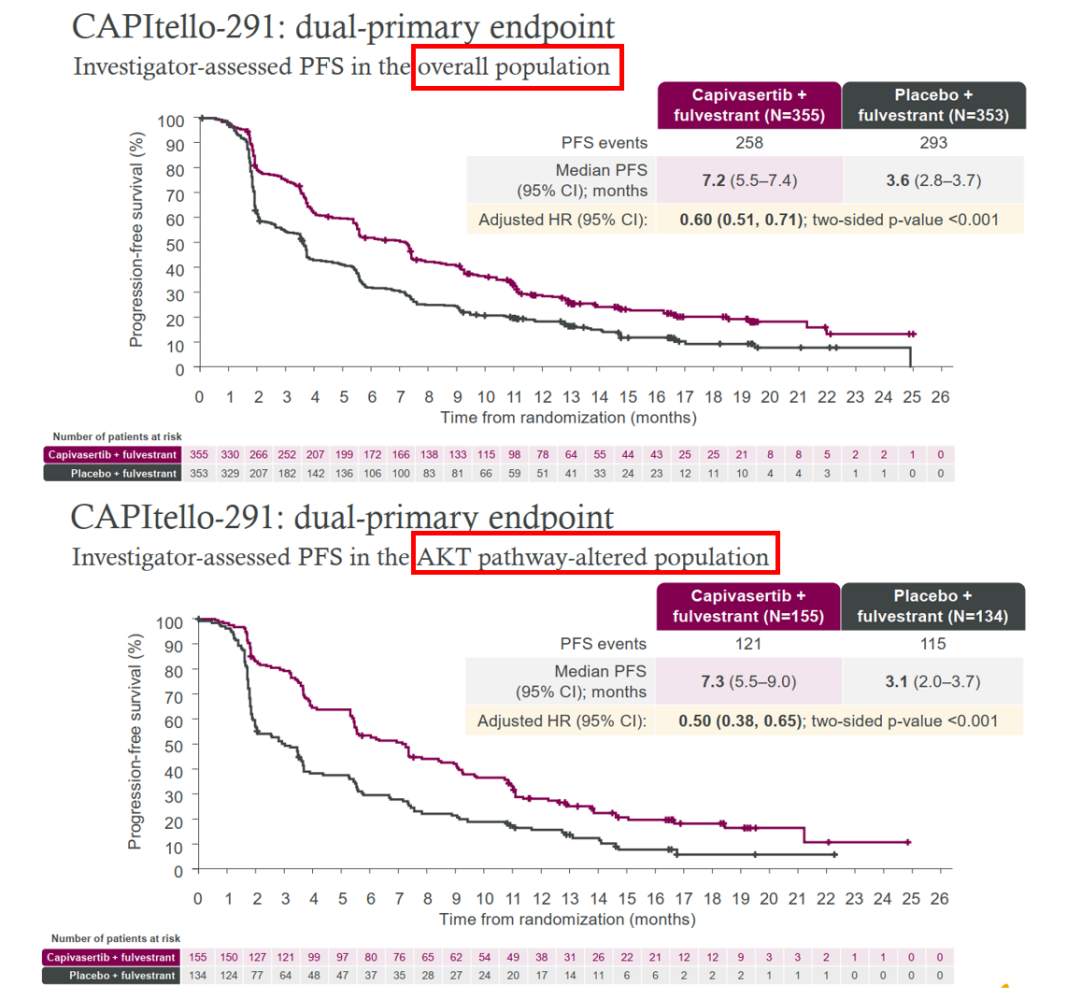
Figure 4 CAPItello-291 meets dual primary endpoints in phase 3 clinic
2) Camizestrant SERENA-2, a next-generation oral selective estrogen receptor degrader, met the primary endpoint in a Phase 2 clinical trial. In postmenopausal women with ER+/HER2-ABC, SERENA-2 at doses of 75 and 150 mg improved PFS better than fulvestrant in postmenopausal women with ER+/HER2-ABC. Safety-wise, both doses of SERENA-2 were well tolerated, with infrequent grade ≥3 TRAEs, dose reductions and discontinuations.
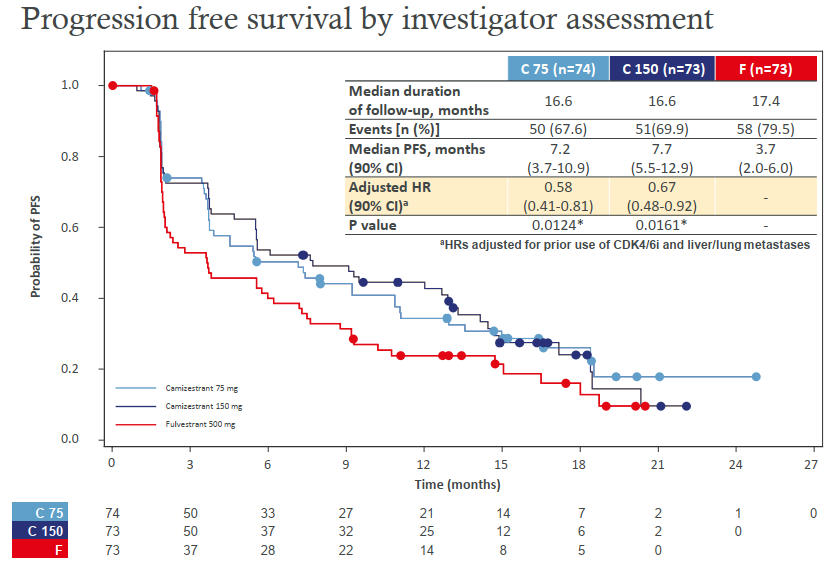
Figure 5 Camizestrant SERENA-2 Phase 2 clinical trial results
In addition to CAPItello-291 and SERENA-2, there has been positive progress in the development of drugs such as Enhertu and the next generation ADC class, Dato-DXd.
Cardiovascular disease drug development
AstraZeneca's research areas include heart failure, cardiovascular disease, diabetes, non-alcoholic steatohepatitis (NASH) and chronic kidney disease. the global burden of CVRM disease on the human, economic and healthcare systems is staggering.
With increasing obesity rates and an ageing population, CVRM is set to increase dramatically over the next decade. However, with only three drugs currently in Phase 3 clinics, AstraZeneca urgently needs to expand its presence in CVRM and therefore the acquisition of CinCor is in line with the company's long-term strategic layout.
Respiratory and immunology-related drug development
AstraZeneca's main focus is on chronic respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), as well as immune-driven diseases such as lupus. There are currently four relevant drugs in clinical phase I, seven in phase II and six in phase III.
Rare disease area
For 30 years, AstraZeneca's pioneering science has led to the development of transformative medicines for rare diseases, but rare disease research and development presents unique challenges that require continuous innovation in order to deliver meaningful medicines to patients.
Driven by a patient-centred approach, AstraZeneca is paving the way by adapting elements of the R&D process and developing new tools and approaches to gain a deeper understanding of many rare diseases and patient needs. Currently, AstraZeneca is present in rare disease areas such as transthyretin amyloid cardiomyopathy and paroxysmal nocturnal haemoglobinuria.
In addition to these major disease areas, AstraZeneca has a presence in vaccines, immunotherapy, osteoarthritis pain and painful diabetic neuropathy.







